Background: Owing to the unique therapeutic characteristics and diverse etiology of underlying disease, navigating available regulatory guidance can be a challenge for newcomers to therapeutic research and development (R&D). Further, classes of drugs and biologic products are regulated separately by the U.S. Food and Drug Administration (FDA) centers CBER and CDER, respectively.
Methods: We provide background on the FDA guidance structure herein and further examine publicly registered clincialtrials.gov data to provide an overview of development status by phases and novel modality (i.e., CAR-T) in context of the regulatory landscape.
Results: Therapeutic products developed for malignant hematological disease can be regulated by either CDER or CBER depending on the type of drugs, ranging from small molecules to macromolecules, including cellular and gene therapies. In 2019, CDER of Hematology and Oncology Products (OHOP) has been reorganized and renamed the Office of Oncologic Diseases (OOD). The new OOD structure consists of six divisions including Division of Hematologic Malignancies 1 (DHM1) and Division of Hematologic Malignancies 2 (DHM2) for the review of hematologic malignancies. In 2022, CBER of Office of Tissues and Advanced Therapies (OTAT) was transformed into the Office of Therapeutic Products (OTP) to address the exponential growth in cell and gene therapies. PDUFA VII expanded the INTERACT meeting program to include both centers, a major change that may drive early phase development collaboration.
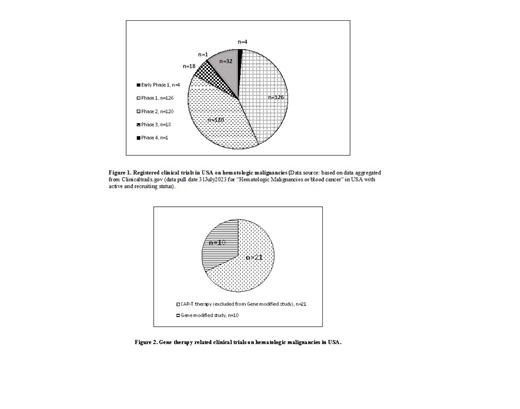
A review on the current state of hematologic malignancies clinical trials in USA shows that, of 301 hematologic malignancies related trails in USA registered to ClinicalTrials.gov (Fig. 1), 4 are in early phase 1(exploratory phase), 126 in phase 1, 120 in phase 2, 18 in phase 3, and 1 in phase 4. Most of the trials are still in early phases 1 or 2. Among the 301 registered clinical trials, 21 of clinical trials (Fig. 2) are CAR-T related, and 10 of the trials are gene modified cell products related.
Conclusions: Compared with traditional treatment on malignant hematological disease, immunotherapy is the upcoming trend in cancer treatment. Chimeric antigen receptor (CAR) therapy is at the forefront of cellular/adoptive immunotherapy for cancer treatment due to its sustained remission and better quality of life. Much research and clinical trials have been undertaken in recent years which led to several successful outcomes in CAR therapy. Despite tremendous progress, CAR-T are still faced with many challenges, including evolving regulatory framework along the road. We summarize the regulatory landscape to assist therapy developers to align with FDA expectations.
Disclosures
Wang:Cytiva: Current Employment. Johnson:Cytiva: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal